SURGICAL TREATMENT
 Operative Procedure
Operative Procedure
The best route of approach will depend on the shape, size, and
direction of growth of the individual craniopharyngioma. The
location of cysts, which often create a path to the solid
portion of the tumor, may also affect the choice of approach.
Certain general principles can be defined. The approach chosen
should offer the greatest exposure of tumor to subarachnoid
pathways at the cranial base. Approaches that do not sacrifice
or divide neural structures are generally preferred to those
that do. Unilateral approaches are to be preferred over those
that mobilize both sides of the brain.
The two approaches most commonly used are the subfrontal and
pterional approaches. Both of these routes allow the surgeon to
approach the tumor below the circle of Willis, with excellent
visualization of the nerves and chiasm. The pterional route is
the shorter approach, and allows good visualization of the
retrochiasmatic area. The subfrontal approach allows excellent
visualization of the anterior portion of the optic pathways, as
well as a direct approach through the lamina terminalis if
required. Both approaches can be subdivided into a number of
different pathways, as follows.
1. Subchiasmatic pathway. This is the traditional approach
between the optic nerves.
2. Opticocarotid pathway. This route, between the internal
carotid artery and the optic nerve and tract, is useful when the
chiasm has been pushed forward by tumor and appears to be
prefixed, with shortened optic nerves.
3. Lamina terminalis pathway. The lamina terminalis may be
exposed above the chiasm and divided between the optic tracts.
Opening the lamina terminalis often does not expose the tumor,
because it is covered by the thinned floor of the third
ventricle. The floor may be opened, or it may be possible to
displace the mass downward ad remove it subchiasmatically or
laterally.
4. Pathway lateral to the carotid artery. Opening the arachnoid
at the rostral end of the sylvian fissure and retracting the
temporal pole may give access to the lateral tumor surface.
5. Transfrontal-trans-sphenoidal pathway. It requires drilling
away the tuberculum sellae, opening the sphenoid sinus, and
removing the anterior sellar wall. This approach is useful when
the chiasm is prefixed and the tumor fills the sella. It may be
employed with opening of the lamina terminalis. I find a more
restricted drilling of the tuberculum to be adequate, and I try
not to enter the sphenoid sinus, instead leaving its mucosa
intact.
6. In case of giant craniopharyngioma with massive
intraventricular extension with the superior cystic part pushing
up the foramen Monro , it is wise to attack the tumor through
interhemispheric transcallosal or transcortical transfrontal
approach directly to the lesion, taking into consideration the
geometry of the tumor and the best visual access to the most
parts of the tumor as in this case.
As the tumor is approached, each of the possible routes is
evaluated, and the greatest possible exposure of the tumor
surface is sought. When the tumor is retrochiasmatic. the chiasm
will appear to be prefixed because of displacement from behind.
Much has been written of "short" optic nerves, but some of the "prefixity"
is lost during the course of operation. Lateral displacement of
the optic tract is due to a similar mechanism, distorting the
tract and decreasing the space between its lateral surface and
the carotid artery.
When a portion of the tumor is exposed, the arachnoid covering
the tumor is carefully opened. Care is taken not to coalesce the
arachnoid with the tumor capsule by using the cautery, as
preservation of the subarachnoid CSF plane is necessary for
safe and total tumor removal. A needle is inserted into the
tumor and the cyst is aspirated. All tumors should be aspirated,
even ones that appear solid on MRI or CT, since the latter may
be partially cystic, and removal of only I or 2 ml may provide
room for dissection. Entering the tumor with a small
microsuction tip will further decompress the tumor. As the
tumor mass decreases, more of the capsule can be exposed by
dissecting in the subarachnoid CSF plane around the tumor. Small
arterial feeders from the anterior circulation to the tumor may
be coagulated and divided, remembering that the plexus of small
arterial feeders to the undersurface of the chiasm and optic
tracts must be spared. Fortunately, little blood supply is
derived from the posterior portion of the circle of Willis, and
the caudal surface of these tumors is usually, but not always,
less adherent. The interior of the tumor is entered, and the
solid portion removed piecemeal. As these fragments are removed,
care must be taken to protect the optic nerve and tract,
especially from the calcified fragments. Larger calcifications
should be crushed to an appropriate size for removal. The
internal carotid artery must be protected as well, and fusiform
dilations of the carotid on the operative side have been
reported. As the tumor is progressively gutted, portions of the
capsule can be resected. It is important not to "lose the
handle," however, as the capsule would then retract upward out
of sight and might prove impossible to retrieve. After the
attachments of the tumor to the optic apparatus, hypothalamus,
and basal arterial vessels have been dissected with higher
microscopic magnification, the tumor remnant is usually easily
delivered. Small angled dental mirrors are then used to inspect
the undersurface of the chiasm and the median eminence region
for tumor remnants.
 Alternative Approaches
Alternative Approaches
The trans-sphenoidal route has been widely employed for removal
of tumors that are largely intrasellar or have only small
suprasellar components. Larger tumors, with greater suprasellar
extension, may be removed by those skillful in this approach. A
suprasellar extension with a smooth contour may indicate an
intact diaphragma sellae that is stretched upward by the tumor
mass. The trans-sphenoidal route is often best in such a case,
as it allows direct access to the tumor while the intact
diaphragma helps protect the undersurface of the brain.
In one series of trans-sphenoidal operations, many of the tumors
involved had little in the way of sellar enlargement. An
anteriorly displaced pituitary gland was a frequent finding,
necessitating division of the pituitary gland to achieve tumor
removal with preservation of endocrine function.
This approach is not applicable when the sella is normal in
size, when pituitary function is totally intact, or if the
suprasellar portion of the tumor extends laterally or anteriorly
away from a direct line of approach through the sella. Long-term
follow-up studies after trans-sphenoidal removal have shown both
good control of tumor recurrence and surprisingly good endocrine
function. Laws has described a case in which the removal of a
densely calcified tumor trans-sphenoidally resulted in damage to
both carotid arteries and death.
The transcallosal approach may be chosen for tumors that lie
largely in the third ventricle. It is most useful when the
intraventricular portion is solid or calcified or when no
portion of the tumor can be visualized in the basal subarachnoid
pathways. A number of cases in which the craniopharyngioma was
totally within the third ventricle have been described. The
transcallosal approach for craniopharyngioma was first reported
in 1973. Since then this operative approach has become
increasingly familiar to neurosurgeons, and its technique has
been well described.
This approach is most useful when the foramen of Monroe is widely
dilated, either by the tumor or by hydrocephalus. When the
transforaminal route is not available, it may be necessary to
use either the interforniceal approach described by Apuzzo or
the subchoroidal approach. It is better to avoid enlargement of
the foramen of Monroe by dividing the fornix, because of concern
about memory problems. Two vascular problems have arisen in the
use of this approach. The first is vasospasm of the anterior
cerebral arteries as they are dissected and retracted; the
second is damage to or obstruction of the deep venous system as
the third ventricle is entered.
 Preserving the Pituitary Stalk
Preserving the Pituitary Stalk
The pituitary stalk is necessary for the resumption of normal
pituitary responses, and its preservation is now possible with
microscopic visualization. Even if the stalk is damaged, a
remnant that reaches from the median eminence to the pituitary
will serve as a matrix on which the important portal system may
reform. Recognizing the stalk is basic to preserving it. Under
higher magnification, the stalk has a striate pattern that is
distinctive among neural structures. This striation is caused by
the parallel arrangement of the long portal veins and is
maintained despite severe distortion of the stalk. Once the
stalk is visualized, it is often possible to dissect the tumor
away without sacrificing the stalk. While the stalk has been
described as lying on the posterior surface of the tumor, it may
be found displaced laterally or anterolaterally as well.
Patients with an intact stalk appear to regain endocrine
function more quickly and complete than other patients, and a
case has been described of total removal without postoperative
diabetes insipidus.
 Staged Procedures
Staged Procedures
Occasionally it is desirable or necessary to perform a two-stage
procedure to achieve total tumor removal. Koos and Miller have
described a procedure in which intracranial removal of the
suprasellar portion of the tumor was followed by trans-sphenoidal
removal of intrasellar tumor at a later operation.
Some tumors have extensions that make removal by any single
route hazardous or unfeasible. When the tumor has large
suprasellar, retrosellar, and third-ventricle components. A
portion of the tumor has pushed laterally through the choroid
fissure, and a large component is located in the temporal horn.
Midline portions of the tumor are removed via a subfrontal
approach at first operation, and the temporal cyst was aspirated
through its medial surface. The calcified portion in the
temporal horn and a large calcified interpeduncular fragment are
excised in a subsequent transtemporal procedure.
 False Cure
False Cure
Virtually every large series of craniopharyngiomas has reported
recurrences of tumor after "total" removal. Amacher has reviewed
several series and has documented 17 recurrences after 92 total
removals. Other reports show that even use of the operating
microscope and careful examination with micromirrors may not
prevent the operator from thinking that total removal has been
achieved when it has not. Routine use of MRI postoperatively
will reveal many, if not all, of these residual tumor fragments.
When postoperative MRI is required as a criterion of total
removal, the false-cure rate can be expected to fall.
 Postoperative Management
Postoperative Management
Most patients who undergo total or radical subtotal tumor
removal will have either temporary or permanent disruption of
neurohypophyseal axis function. Damage to the stalk or pituitary
may result in various endocrine deficiencies, of which loss of
adrenocorticotropic hormone (ACTH) and antidiuretic hormone (ADH)
will be notable in the immediate postoperative period. Loss of
other endocrine functions is important in the long-term
management of these patients.
Lack of corticosteroid production secondary to loss of ACTH
production is rarely a problem in patients who are receiving
large doses of high-potency synthetic corticosteroids, which are
used in most centers to control cerebral swelling. Because these
synthetic steroids have little mineralocorticoid effect, some
workers have advocated using cortisone acetate in physiological
doses in addition to the high-potency agents. As the risk of
edema lessens, the synthetic steroids are tapered off, and
cortisone replacement at a physiological dosage is given. These
patients must be regarded as being hypoadrenal at all times, and
death of a patient during a metyrapone test has been reported.
Diabetes insipidus is noted shortly after the operation but may
begin during the surgical procedure; it is best managed
initially by fluid replacement. If excessive thirst or fluid
replacement problems become difficult for the patient,
vasopressin may be given, preferably in a short-acting form.
Patients who have diabetes insipidus due to stalk sectioning may
have subsequent involution of neurohypophyseal axons or
infarction of a portion of the pituitary, caused by interruption
of the blood supply from the portal vessels. ADH may then be
released from the degenerating axon terminals in
superphysiologic amounts. When these events occur, they usually
take place from 48 to 96 h after stalk damage. Patients who have
been given long-acting vasopressin may be at risk for renal
shutdown under these circumstances. Experience at many centers,
including mine, with synthetic DDAVP (desmopressin acetate)
spray has been satisfactory. Determinations of fluid intake and
output, urine specific gravity, and rate of urinary excretion at
2-h or 3-h intervals are helpful in the immediate postoperative
period. Daily or twice-daily blood counts, serum electrolyte
determinations, and accurate patient weight measurements are
also needed. Operative deaths are usually attributed to
hypothalamic injury, which causes a clinical syndrome
characterized by hyperpyrexia and somnolence. Damage to
osmoreceptors in the anterior hypothalamus may lead to loss of
the sensation of thirst. Patients are then difficult to manage,
as thirst is a major factor in the treatment of the concomitant
diabetes insipidus. Other postsurgical hypothalamic deficits may
include disturbance of caloric balance, changes in wakefulness,
changes in affective behavior, and disturbances of memory.
 Author's approach:
Author's approach:
The author prefer using combined bifrontal subfrontal approach
with pterional modification according to the location of the
tumor. Mobilization and preservation of the olfactory tracts is
performed routinely. Inspection of 220 degrees around the
chiasmal region is possible, giving the surgeon all the
abilities to perform all needed maneuvers. For demonstration
click here!
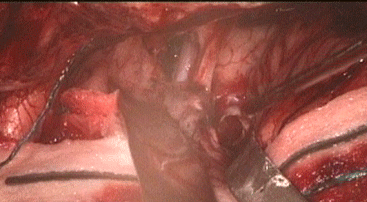
Photo showing the cavity medial to the left olfactory trigon and
the empty space after removal of the solid mass between the ICA
and the left oculomotor nerve. It was possible to see the
basilar artery inside the cavity.
|